By continuing to use this site, you agree to our use of cookies as described in our Cookie Policy.

Immediate-release granules

Extended-release pellets
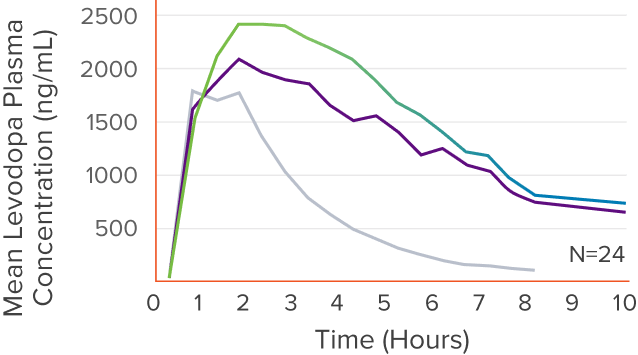
*In a clinical pharmacology study, the duration of levodopa levels was defined by how long the levels of CREXONT, RYTARY, and IR CD/LD were maintained in the blood above half the maximum concentration.
†This was a post hoc analysis of a secondary measure in the clinical pharmacology study; CREXONT vs RYTARY P=0.010; CREXONT vs IR CD/LD P<0.0001.
More “Good On” time

MAIN MEASUREMENT
compared with IR CD/LD
‡“Good On” time is defined as “On” time without troublesome dyskinesia. Change in “Good On” time was measured by comparing values at the end of study to baseline; P=0.019 vs IR CD/LD.
§This was the average dose frequency during the double-blind maintenance period.
SECONDARY MEASUREMENT
compared with IR CD/LD
||This was a secondary endpoint of the study.
¶Study end=Week 20 or early termination; P=0.025 vs IR CD/LD.
A DIFFERENT ANALYSIS OF THE SAME STUDY
compared with IR CD/LD
#“Good On” time is defined as “On” time without troublesome dyskinesia.
**This was a post hoc analysis of the study; P<0.0001 vs IR CD/LD.

MAIN MEASUREMENT
30 mins more “Good On” time with less frequent dosing‡§
‡“Good On” time is defined as “On” time without troublesome dyskinesia. Change in “Good On” time was measured by comparing values at the end of study to baseline; P=0.019 vs IR CD/LD.
§This was the average dose frequency during the double-blind maintenance period.
SECONDARY MEASUREMENT
30 mins less “Off” time per day||¶
People who took CREXONT had 30 minutes less “Off” time per day compared with those who took IR CD/LD
||This was a secondary endpoint of the study.
¶Study end=Week 20 or early termination; P=0.0252 vs IR CD/LD.
People who took CREXONT had 30 minutes less “Off” time per day compared with those who took IR CD/LD
A DIFFERENT ANALYSIS OF THE SAME STUDY
#“Good On” time is defined as “On” time without troublesome dyskinesia.
**This was a post hoc analysis of the study; P<0.0001 vs IR CD/LD.
CREXONT® (carbidopa and levodopa) extended-release capsules is a prescription medication for the treatment of Parkinson’s disease, Parkinson’s disease caused by infection or inflammation of the brain, or Parkinson’s disease-like symptoms that may result from carbon monoxide or manganese poisoning in adults.
Do not take CREXONT with antidepressant medications known as nonselective monoamine oxidase (MAO) inhibitors.
Do not take CREXONT with other carbidopa-levodopa preparations without consulting your healthcare provider.
CREXONT may cause falling asleep during activities of daily living, somnolence, or dizziness. Avoid activities that require alertness such as driving and operating machinery until you know how CREXONT affects you.
The most common side effects that may occur with CREXONT are nausea and anxiety.
It is important to avoid sudden discontinuation or rapid dose reduction of CREXONT. If you are discontinuing CREXONT, work with your healthcare provider to taper the dose over time to reduce the risk of fever or confusion.
You may take CREXONT with or without food, but taking it with food may decrease or delay its effect. Consider taking the first dose of the day about 1 to 2 hours before eating.
Swallow CREXONT whole. Do not chew, divide, or crush the capsules.
Do not take CREXONT with alcohol.
Tell your healthcare provider if you:
CREXONT® (carbidopa and levodopa) extended-release capsules is a prescription medication for the treatment of Parkinson’s disease, Parkinson’s disease caused by infection or inflammation of the brain, or Parkinson’s disease-like symptoms that may result from carbon monoxide or manganese poisoning in adults.
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Specialty, a division of Amneal Pharmaceuticals LLC at 1-877-835-5472 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please read the full Prescribing Information. For more information talk to your healthcare provider.
CREXONT® (carbidopa and levodopa) extended-release capsules is a prescription medication for the treatment of Parkinson’s disease, Parkinson’s disease caused by infection or inflammation of the brain, or Parkinson’s disease-like symptoms that may result from carbon monoxide or manganese poisoning in adults.
Do not take CREXONT with antidepressant medications known as nonselective monoamine oxidase (MAO) inhibitors.
Do not take CREXONT with other carbidopa-levodopa preparations without consulting your healthcare provider.
CREXONT may cause falling asleep during activities of daily living, somnolence, or dizziness. Avoid activities that require alertness such as driving and operating machinery until you know how CREXONT affects you.
The most common side effects that may occur with CREXONT are nausea and anxiety.
It is important to avoid sudden discontinuation or rapid dose reduction of CREXONT. If you are discontinuing CREXONT, work with your healthcare provider to taper the dose over time to reduce the risk of fever or confusion.
You may take CREXONT with or without food, but taking it with food may decrease or delay its effect. Consider taking the first dose of the day about 1 to 2 hours before eating.
Swallow CREXONT whole. Do not chew, divide, or crush the capsules.
Do not take CREXONT with alcohol.
Tell your healthcare provider if you:
CREXONT® (carbidopa and levodopa) extended-release capsules is a prescription medication for the treatment of Parkinson’s disease, Parkinson’s disease caused by infection or inflammation of the brain, or Parkinson’s disease-like symptoms that may result from carbon monoxide or manganese poisoning in adults.
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Specialty, a division of Amneal Pharmaceuticals LLC at 1-877-835-5472 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please read the full Prescribing Information. For more information talk to your healthcare provider.
CREXONT® is a registered trademark of Amneal Pharmaceuticals LLC. © 2025 Amneal Pharmaceuticals LLC.
Distributed by Amneal Specialty, a division of Amneal Pharmaceuticals LLC.
All rights reserved. PP-IPX203-US-0026 06/2025
This is intended for residents of the United States only. Any product discussed herein may have different product labeling in different countries. Please read the full Prescribing Information and discuss it with your doctor or healthcare professional.